Abstract

Introduction: Whether immune checkpoint inhibitors (ICIs) are associated with an increased risk of venous thromboembolism (VTE) compared to traditional chemotherapy (chemo) is unclear (PMID: 34572833). While retrospective cohort studies reported higher than expected rate of VTE among patients receiving ICI, these patients were often sicker and having received multiple prior lines of therapy. In recent years, ICIs have been used increasingly in the front line setting for several cancers. In the current study, we assessed the incidence of VTE associated with first-line ICIs vs. chemo among comparable cancer patients.
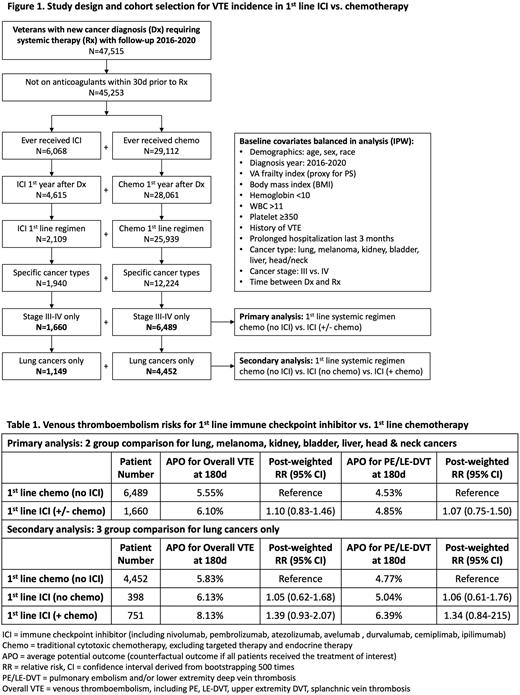
Methods: We performed a retrospective cohort study using the national Veterans Affairs (VA) healthcare system. Patients with de novo incident cancer diagnosis 2016-2020 were identified from the VA Cancer Registry and linked to the VA Corporate Data Warehouse. Patients were excluded if they had benign histology, were not primary VA user, or did not receive systemic therapy. For the primary analysis (chemo vs. ICI +/- chemo), we restricted the population to patients with stage III-IV cancers of lung, skin (melanoma), kidney, bladder, liver (hepatocellular carcinoma), and head/neck who received first-line ICI or first-line chemo within first year of diagnosis (Figure 1). For the secondary analysis (chemo vs. ICI vs. ICI/chemo), we further restricted the population to those with lung cancers as concurrent first-line ICI/chemo administration was only observed in this cohort. Incident VTE and PE/LE-DVT were defined using our computable phenotype algorithm (PMID: 35647478). When tested against the gold standard of chart abstractor review in a random sampling of 400 patients, the algorithm had a positive predictive value of 90% and weighted sensitivity of 82%.
The following covariates were extracted at the time of first-line ICI or chemo (index date): age, sex, race, diagnosis year, VA frailty index (proxy for performance status), history of VTE, recent prolonged hospitalization last 3 months, cancer type, cancer stage, time delay between diagnosis and treatment, and other Khorana Score components (body mass index, hemoglobin, white blood cell count, platelet count). To address confounding, we used inverse probability weighting (IPW) with overlap weights by fitting a propensity score (PS) logit model from these baseline covariates (2 groups for primary analysis, 3 groups for secondary analysis). Average potential outcome (APO) for VTE and PE/LE-DVT were estimated at 6-months. Weighted relative risk (RR) with bootstrapped 95% confidence interval (CI) were calculated using weighted logistic regression. IPW analyses were performed using the PSweight package in R.
Results: From 2016-2020, 45,253 veterans with new cancer diagnosis requiring systemic therapy met the inclusion criteria of whom 6,068 (13.4%) received ICI with a median continuous follow-up of 334 days. However, only 4,615 (10.2%) received ICI within first year of diagnosis, 2,109 (4.7%) received ICI as the initial regimen, and 1,660 (3.7%) received it for FDA-approved cancers (vs. 6,489 comparable chemo patients) (Figure 1). Despite initial cohort matching, the groups remained imbalanced with standardized mean difference (SMD) >0.1 in age, diagnosis year, cancer type, cancer stage, and time to therapy. After IPW, all covariates were balanced with SMD <0.1.
In the primary analysis (Table 1), VTE occurred in 5.55% of chemo group vs. 6.10% of ICI group at 6 months. The weighted RR was 1.10 (95% CI 0.83-1.46) for ICI (+/- chemo) vs. chemo (no ICI). In the secondary analysis restricted only to lung cancers, VTE occurred in 5.83% of chemo group vs. 6.13% of ICI group (no chemo) vs. 8.13% of ICI group (+ chemo) at 6 months. The weighted RR was 1.05 (95% 0.62-1.68) for ICI (no chemo) vs. chemo (no ICI). The weighted RR was 1.39 (95% 0.93-2.07) for ICI (+ chemo) vs. chemo (no ICI). The PE/LE-DVT outcome followed a similar trend.
Conclusions: In this PS-weighted analysis of 8,149 patients, we found that first-line ICI and chemo had a similar rates of VTE. Larger studies are needed for the subgroup of lung cancer patients where combination ICI/chemo regimen had a non-significant 39% increase in VTE rate compared to either chemo or ICI alone. Previous retrospective studies likely reported inflated rate of VTE in ICI due to indication bias. Nonetheless, patients receiving ICI had a similar rate of VTE and should be risk stratified as those receiving chemo.
Disclosures
Carrier:Pfizer: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy; Servier: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Leo Pharma: Consultancy, Honoraria, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal